A Guide to Diamond Types and Why They Matter
From nitrogen to boron, the classification of diamond types explains the hidden science behind a diamond’s color and authenticity.

When it comes to diamonds, most of us are familiar with cut, color, clarity, and carat weight. Those four characteristics describe how a diamond looks and help determine its value. But behind the scenes, gemologists rely on another system that explains why diamonds look the way they do and helps determine whether a diamond is natural, color-treated, or lab-grown. That system is called diamond typing.
Diamond typing can sound technical, but the concept is surprisingly straightforward. According to the Gemological Institute of America (GIA), diamond type classifies diamonds based on their chemical composition and atomic structure. More specifically, the presence or absence of trace elements such as nitrogen or boron. Understanding diamond type helps explain a diamond’s color and how it reacts to treatments.
Meet the Expert

- Grant Mobley is the Jewelry & Watch Editor of Only Natural Diamonds.
- He is a GIA Diamonds Graduate.
- He has over 17 years of jewelry industry experience, starting with growing up in his family’s retail jewelry stores.
What Is Diamond Type?
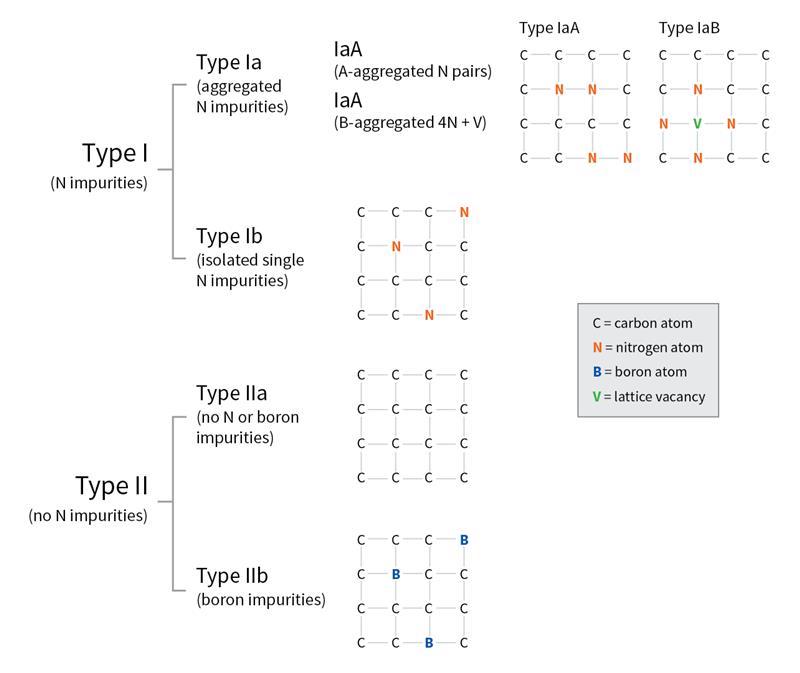
All diamonds consist primarily of carbon atoms arranged in a crystal lattice structure. When a diamond forms deep within the Earth, it usually incorporates trace elements such as nitrogen or boron. Others develop distortions in their atomic structure, known as lattice or optical defects. These microscopic features influence how a diamond absorbs light, which affects its color.
According to the GIA, diamond type is a classification system based on whether nitrogen or boron is present and/or how those elements are arranged within the crystal lattice.
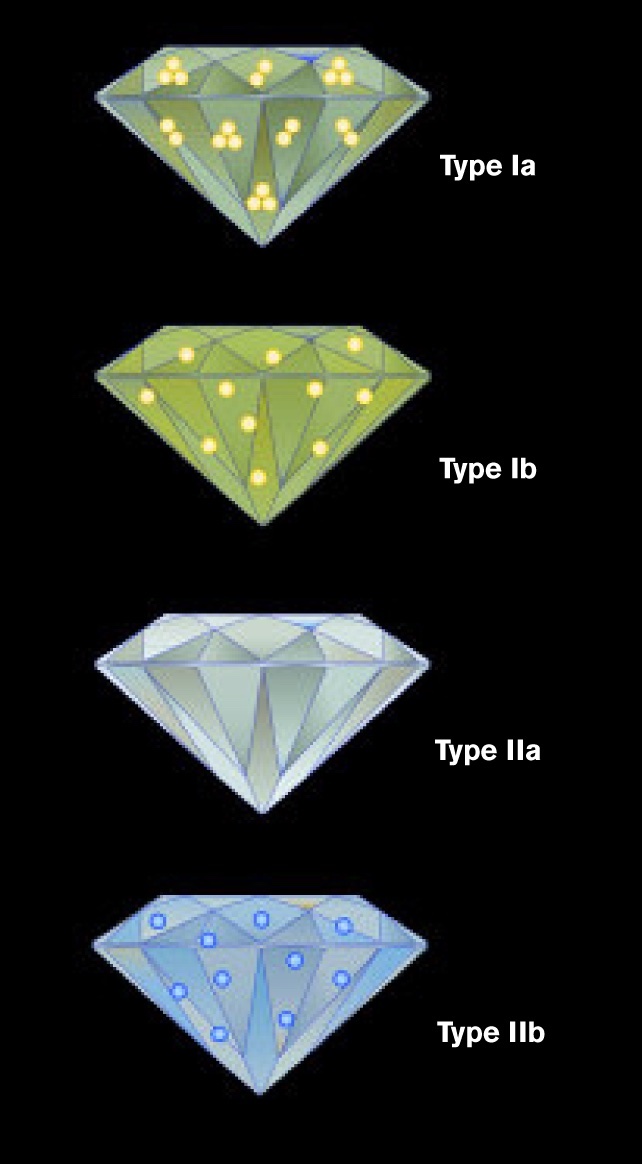
Scientists first introduced diamond types in the 1930s, dividing diamonds into Type I and Type II. Today, gemologists further subdivide these categories into Type Ia, Type Ib, Type IIa, and Type IIb. Many natural diamonds contain a mix of types within a single stone.
Type I Diamonds: Nitrogen Present
Type Ia Diamonds
Type Ia diamonds contain nitrogen atoms grouped in clusters. According to the GIA, approximately 95 percent of all natural diamonds fall into this category. These diamonds usually appear near colorless to light yellow.
Type Ia diamonds subdivide into:
Type IaA, where nitrogen atoms form pairs that do not influence color
Type IaB, where nitrogen atoms form larger aggregates that create a yellow or brown tint
Most diamonds belong to this group. The widespread nature of nitrogen clusters explains why faint warmth appears so commonly in natural diamonds.
Type Ib Diamonds
Type Ib diamonds also contain nitrogen, but in a very different arrangement. Instead of clusters, nitrogen atoms remain isolated throughout the lattice. This structure allows Type Ib diamonds to absorb green light in addition to blue, producing a more intense yellow and sometimes brown color.
Type Ib diamonds account for only about 0.1 percent of natural diamonds, making them extremely rare. Their saturated color and rarity give them strong collector appeal.
Type II Diamonds: No Nitrogen
Type IIa Diamonds
Type IIa diamonds contain no measurable nitrogen or boron, making them the chemically purest diamonds found in nature. They represent roughly 1 to 2 percent of natural diamonds, according to the GIA.
Most Type IIa diamonds appear colorless, but some display gray, light brown, yellow, pink, red, or purple hues. These colors do not come from impurities but from structural distortion caused by extreme pressure during the diamond’s journey toward the Earth’s surface.
High-pressure, high-temperature treatments can sometimes reduce or remove color in these diamonds, making type identification especially important for detecting whether the color is natural.
Type IIb Diamonds
Type IIb diamonds rank among the rarest diamonds on Earth, representing about 0.1 percent of natural diamonds. These diamonds contain boron, which can allow them to conduct electricity and absorb red, orange, and yellow light. As a result, they appear blue or grayish blue.
Some Type IIb diamonds show very subtle boron levels and can appear nearly colorless, but most display unmistakable blue tones. The world’s most famous blue diamonds fall into this category, and their rarity places them among the most valuable diamonds ever discovered.
Why Diamond Type Matters
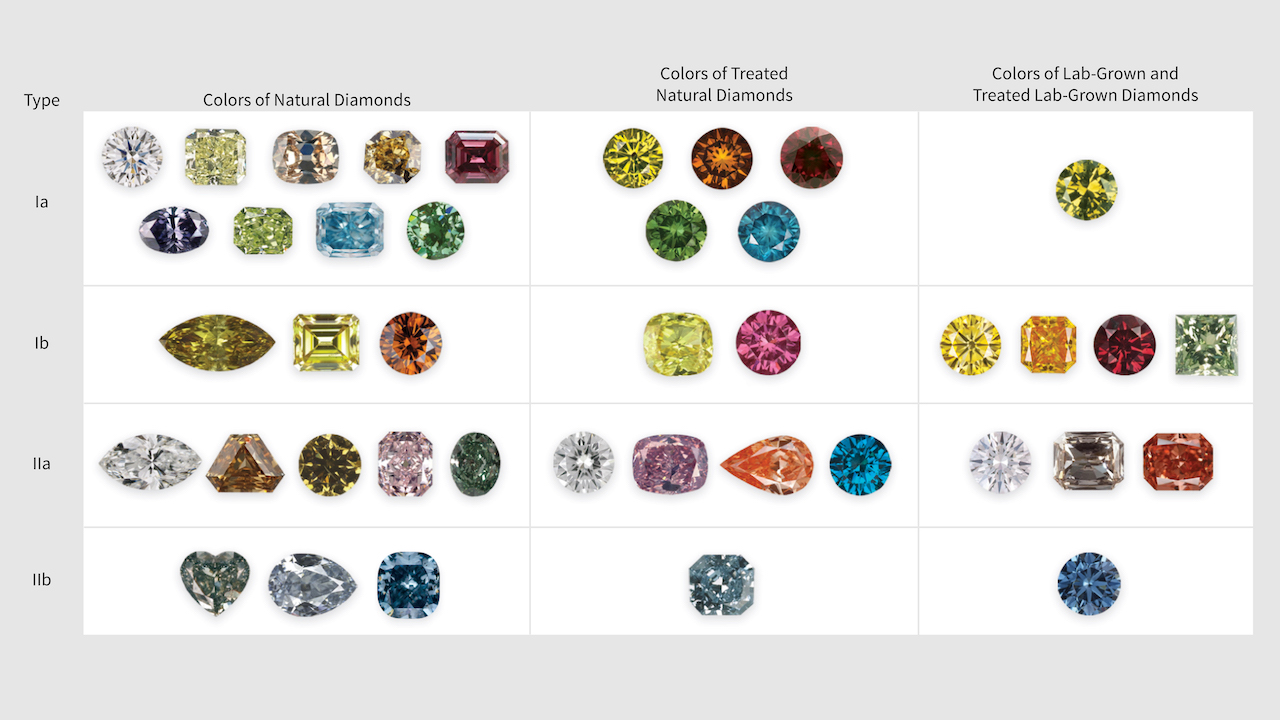
Diamond type plays a crucial role in gemology because it helps professionals determine whether a diamond is natural, treated, or synthetic. Different diamond types respond differently to enhancement techniques, including HPHT treatment and irradiation.
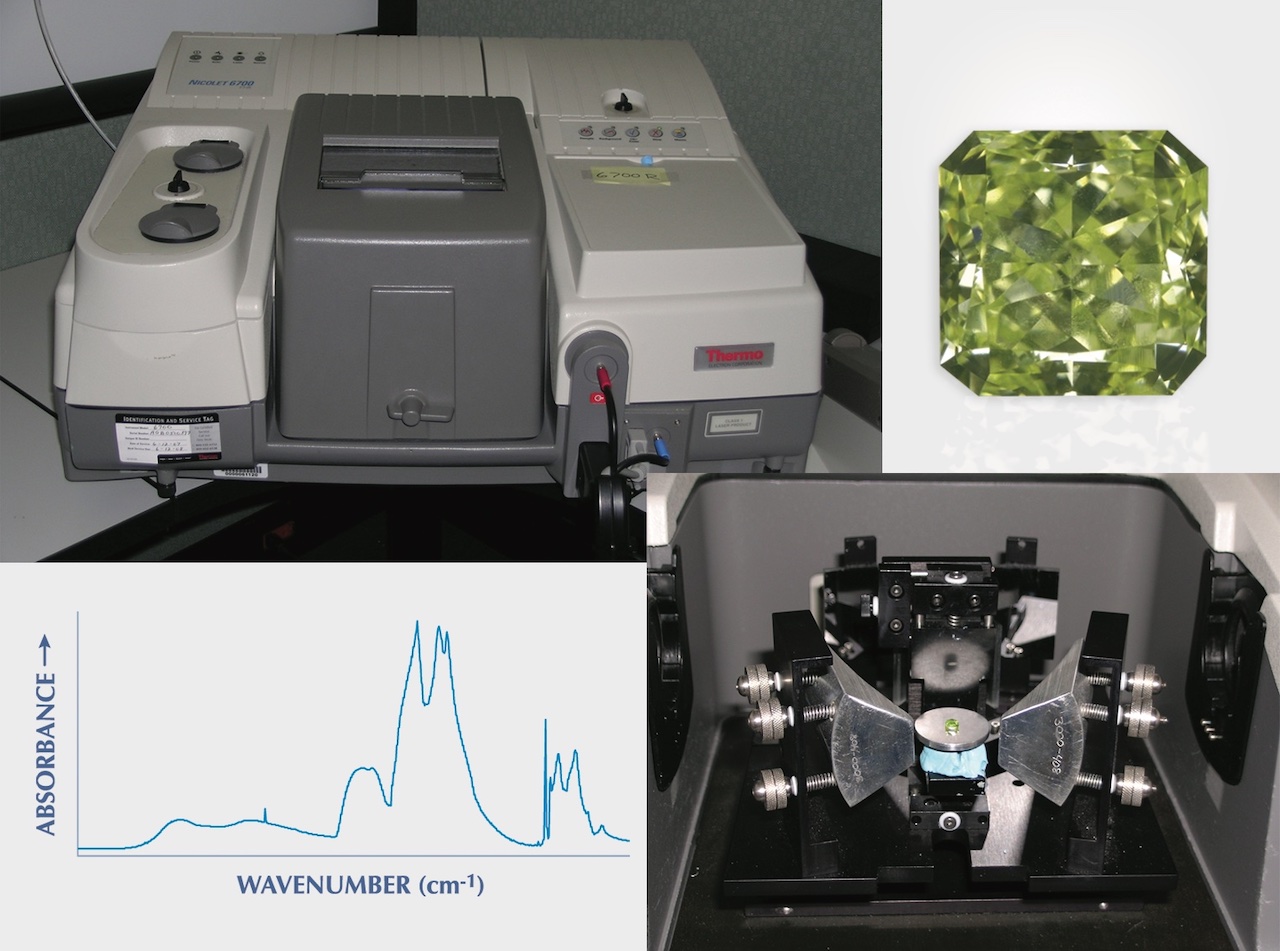
According to the GIA, laboratories determine diamond type using advanced instruments like FTIR spectroscopy. However, trained gemologists can often identify strong indicators using traditional tools such as microscopes and UV lamps.
Diamond type also explains why certain diamonds show unusual colors, react strongly to ultraviolet light, or behave differently during cutting and setting. For collectors and consumers, this knowledge adds transparency and confidence to a purchase.
What Diamond Types Mean for Buyers
You won’t see diamond type listed on most retail grading reports, but it influences everything from color to rarity to value. Diamond typing does not replace the 4Cs. Instead, it complements them by revealing the science beneath the surface. It reminds us that every natural diamond carries a unique atomic story shaped over billions of years.











