< Science of Diamonds / Colored Diamonds
Blue Diamonds and Their Remarkable Link to Ancient Oceans
By Dr. Evan M. Smith, Updated January 16, 2026
The captivating blue of these gems reveals a fascinating story of boron, seawater, and Earth’s deepest processes.

A blue diamond that was examined as part of the study published in the journal Nature. (Courtesy of GIA)
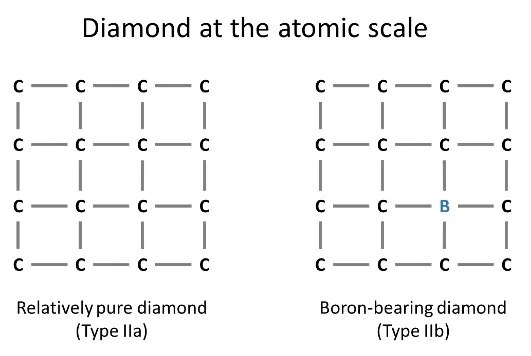
Diamonds come in many colors, including handsome hues of blue. One of the world’s most famous blue diamonds, which has captivated millions of visitors from its pedestal at the Smithsonian, is the Fancy Deep Grayish Blue Hope Diamond. This color comes from trace amounts of boron, which substitutes for carbon in the diamond crystal structure, as shown below.
Some blue diamonds get their color from structural defects produced by radiation exposure or from more complex defects involving hydrogen, but the majority are colored by boron. Scientists have only recently learned that boron-bearing diamonds such as the Hope have an incredible geological backstory.
Meet the Expert

- Dr. Evan M. Smith is a Senior Research Scientist at GIA whose primary research focuses on the geology of diamonds and the inner workings of the Earth.
- Dr. Smith has published in leading academic journals such as Science and Nature, and his findings have been featured by the BBC, The Washington Post, NPR, and other major outlets.
- He holds a Bachelor’s and Master’s degree in Geological Engineering from Queen’s University in Canada, and a Ph.D. in Geology from the University of British Columbia.
- Dr. Smith has been studying diamonds at GIA since 2015.
Ahead, discover how blue diamonds form, why they’re so rare, and the fascinating link they share with ancient oceans.
Blue Diamonds and Type IIb Classification
Boron-bearing diamonds are technically classified as Type IIb. The element boron is abundant at Earth’s surface, but deeper down inside the planet, where diamonds grow, there is very little boron available. Exactly where in the Earth these diamonds crystallize and how they inherit their distinctive punch of boron has remained unknown for decades.
In 2018, the results of an innovative study on Type IIb diamonds was published in the journal Nature. In this study led by GIA, a strategic effort was made to find and examine mineral inclusions such as those shown below. By studying inclusions, which are small pieces of material trapped in the diamond during crystallization, scientists learned about the host rock and depth where Type IIb diamonds form.
How Deep Do Blue Diamonds Form?

Two findings astonished the research team. Firstly, the particular minerals identified in these inclusions are only found at extremely high pressures, very deep in the Earth, leading to the conclusion that Type IIb diamonds formed at least as deep as the so-called transition zone (410–660 km) and perhaps deeper, reaching into the lower mantle (deeper than 660 km). For comparison, this is approximately four times deeper than most other kinds of diamonds, which form near the base of old and thick continents at depths of about 150 to 200 km.
Where Are Blue Diamonds Found?

Unlike many diamonds that form beneath ancient continental cratons, blue diamonds have a distinct geological origin. Most known natural blue diamonds have been recovered from southern Africa, particularly Botswana and South Africa, regions associated with deep mantle-sourced kimberlite eruptions. In fact, Petra Diamonds has just announced the discovery of an almost unbelievable 41.82-carat natural blue diamond from the legendary Cullinan Mine in South Africa. Early images and information from Petra suggest it is of exceptional color and clarity, placing this stone squarely among the most important blue diamond discoveries of all time.
Historically, some of the world’s most famous blue diamonds—including the Hope Diamond and the Wittelsbach—are believed to have originated in India’s Golconda region, though those mines are now exhausted. What unites these disparate locations is not geography, but depth: blue diamonds form far deeper than most other diamonds and are linked to subducted oceanic crust.
Because their formation depends on rare tectonic conditions—where boron-bearing material from ancient oceans is transported hundreds of kilometers into the mantle—blue diamonds are not found wherever diamonds are mined. They appear only where Earth’s deepest geological processes align.
How Many Blue Diamonds Are There in the World?

Blue diamonds are among the rarest diamonds on Earth. Of all natural diamonds mined globally, fewer than 0.02 percent exhibit a blue hue, and only a fraction of those are naturally colored by boron rather than treated or structurally altered.
Even within this already rare category, gem-quality Type IIb blue diamonds are exceptionally scarce. Many researchers estimate that only a few hundred true natural blue diamonds of notable size and saturation exist worldwide. Stones like the Hope Diamond, the Okavango Blue, and the Infinite Blue are therefore not just visually striking—they are geological anomalies formed under conditions that almost never occur.
The Link Between Blue Diamonds and Ancient Oceans
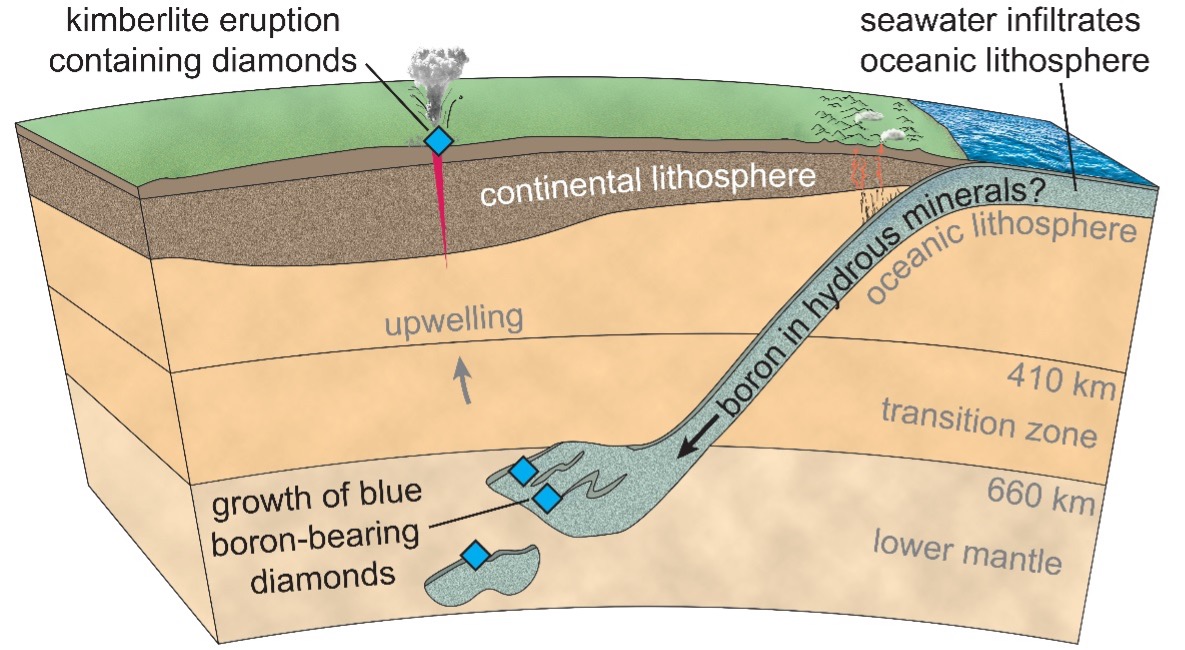
The second exciting observation was that the inclusion mineralogy indicated the diamonds grew in the presence of ocean crust, which was carried down to the lower mantle by the slow, geologic process of subduction. The ongoing descent of subducting oceanic rocks is like a conveyor belt that is capable of drawing surface materials like water, carbon, and boron, down into the interior of the Earth. For Type IIb diamonds, the connection with deeply subducted tectonic plates raises the possibility that the boron atoms contained in these blue gems may have originally been derived from ancient oceans, as shown in the conceptual model below.
Why Do Blue Diamonds Appear Blue?

Now we know that boron-bearing blue diamonds such as the Hope Diamond are among the rare category of diamonds known as “superdeep” diamonds. But why does boron cause color this attractive color to appear at all? Boron modifies the electronic structure in such a way that it will absorb some wavelengths of light.
As long as they are not “compensated” by other defects, the boron atoms in diamond are effectively missing an electron compared to neighboring carbon atoms. It is these uncompensated boron atoms that can interact with and absorb light, especially at particular wavelengths in the infrared spectrum. If the absorption is intense enough, it extends from the infrared part of the spectrum (invisible to us) out into the visible light spectrum, where it preferentially absorbs red light.
The result is a diamond that appears wonderfully blue.














