Everything You Need to Know About Green Diamonds According To A Scientist
Dr. Evan M. Smith of the Gemological Institute of America explains how green diamonds get their colour.
Gemological Institute of America
|
Green diamonds are among the most rare and expensive diamonds to exist. But why is something so breathtaking so hard to come by?
Normally, colour in gemstones is caused by the introduction of additional elements. For example, the substitution of boron or nitrogen atoms for carbon in the diamond crystal lattice can cause blue or yellow colours, respectively.
Green Diamonds are Among The Rarest Diamonds in the World
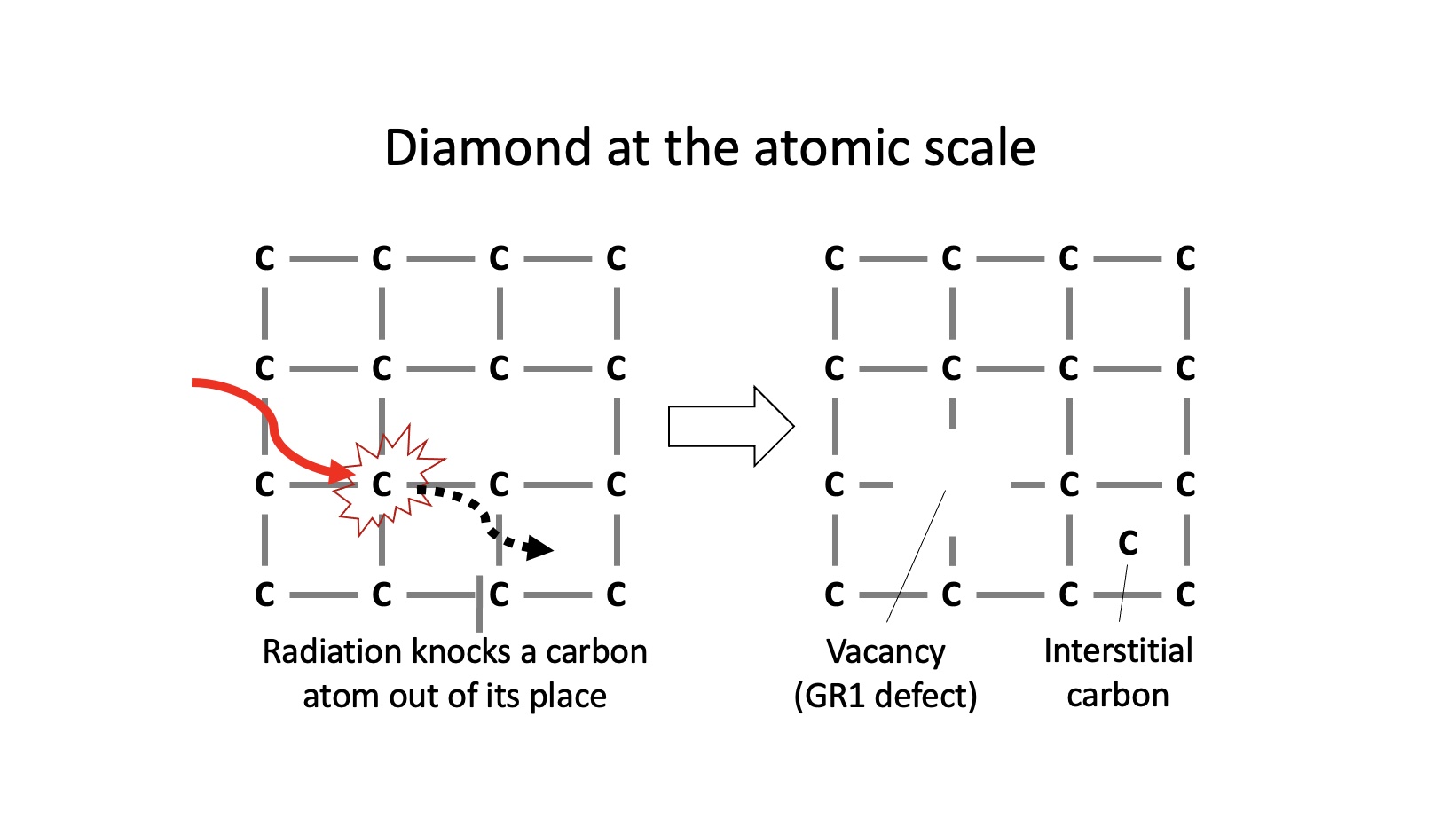
However, when it comes to green diamonds, the colour is often the result of something peculiar: exposure to radioactive minerals or fluids in nature, or artificial irradiation in a laboratory. Such radiation knocks carbon atoms out of place in the crystal structure, as shown below. This generates vacancies, which are essentially vacant sites where the carbon atoms are absent. The cause of colour for many green diamonds is caused by the vacancy defect, also known as the GR1 center in diamond. Other defects involving nitrogen, hydrogen or nickel impurities can also cause green colours, however these are less common.
Natural irradiation happens at Earth’s surface. Think of it as a sort of late stage embellishment to some lucky diamonds, which have already come a long way from their birthplace deep in the mantle. Diamonds that are eroded out of their primary volcanic host rocks and washed down rivers into secondary, alluvial deposits are much more likely to be exposed to naturally occurring radioactive materials. In this geological setting, diamonds can be deposited alongside certain crustal minerals that contain radioactive isotopes of elements such as uranium and thorium. Over thousands or even millions of years, diamonds sitting near these materials are exposed to radiation that can impart a green colour.
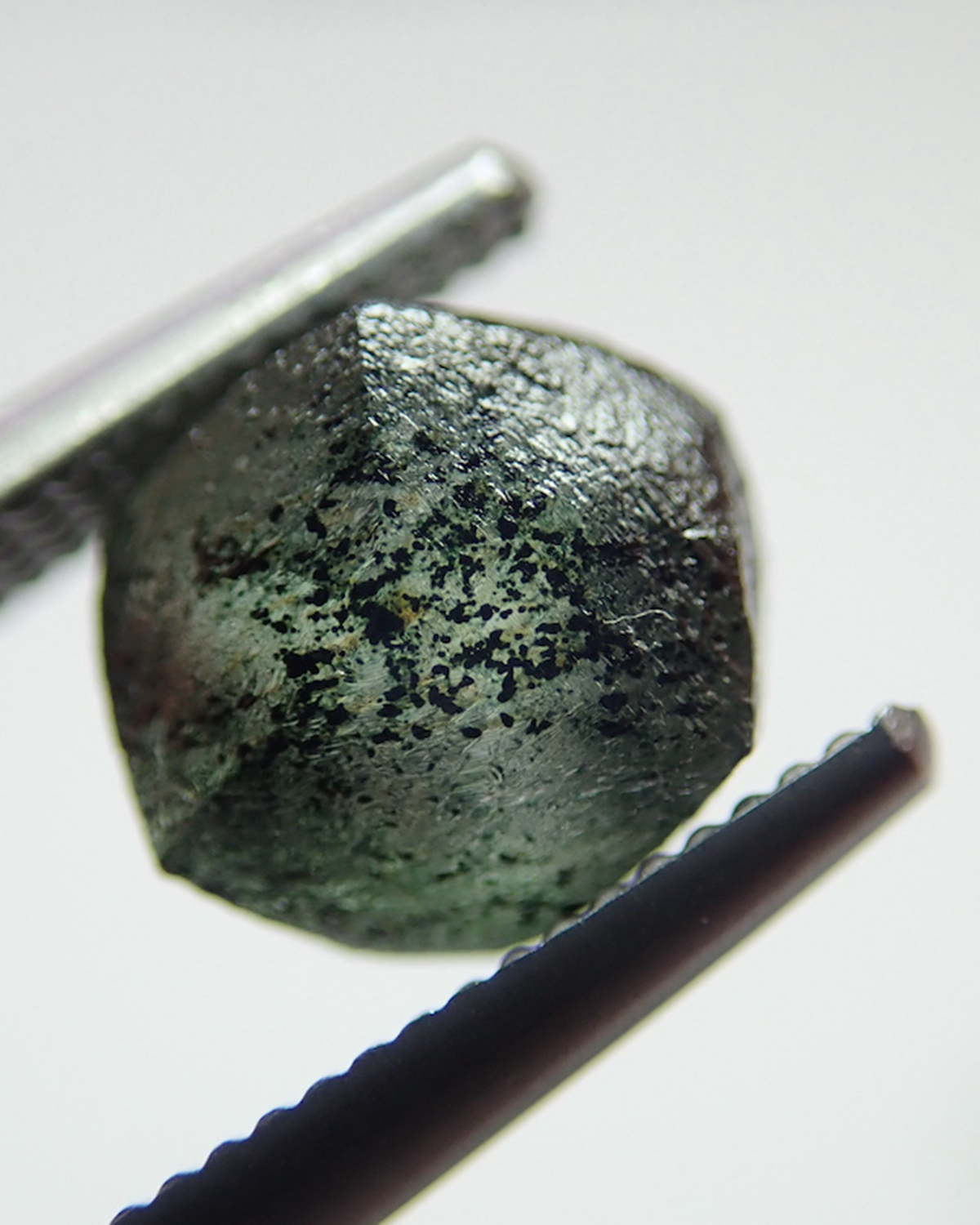
One of the most distinctive features of irradiated diamonds is the presence of conspicuous green “stains” on the rough surface, as seen on the two Brazilian diamonds below. Individual spots mark positions where radioactive mineral grains sat nestled up against the diamond, whereas more irregularly shaped stains might be caused by radioactive fluids or the presence of many fine-grained radioactive particles. This kind of staining is the result of radiation damage by alpha particles, which cannot penetrate very deep into the diamond.
Radiation stains usually only reach a few micrometers into the diamond and this green “skin” can be lost during cutting and polishing. Skilled cutters plan ahead in order to retain the green stains as much as possible, to control the colour and overall faceted appearance. Although stains from alpha decay are relatively shallow, natural irradiation can also come from beta or gamma decay, AKA more energetic forms of radiation that can penetrate farther into the diamond to cause green body colours.

Some radiation stains appear brown, or as a mix of green and brown stains. The brown stains were initially green, but they have deteriorated with natural exposure to heat. Experiments have shown this change can occur quickly at temperatures above 500 to 600 °C, although it may occur at slightly lower temperatures in nature over thousands to millions of years.
It’s important to note that the green colour’s instability at high temperatures raises concerns for polishing; manufacturers must not let the diamond become too hot during the polishing process. A conservative guideline is that if the diamond becomes too hot to touch, the colour is at risk of diminishing. Below, a diamond held firmly in a device called a dop is examined carefully in the course of polishing. The finished 1.42 carat diamond is the result of slow and careful polishing without excessive heating.


While natural irradiation can produce stunning green colours, the process can also be mimicked in a laboratory with electrons, neutrons or gamma rays to generate vacancies and impart a green colour to natural diamonds. And rest assured: the diamond does not carry any kind of residual radioactivity.
In both cases, the physical cause of colour is that same and it has been caused by the same type of process, whether it is natural or artificial irradiation. Though in some instances, a natural colour origin can be concluded based on history, it’s generally difficult to determine where a diamond’s green is from. That said, the colour origins ultimately impacts a green diamond’s value, so advanced testing by a gemological laboratory is crucial.
To this day, many green diamonds in the marketplace are the product of artificial irradiation treatments, which date back to the 1940s. Logic shows that green diamonds documented prior to this time must be natural. A prime example comes from the largest and most famous green diamond, the 41 carat Dresden Green, which was recovered from the historic Golconda mines of India.
